The GST label
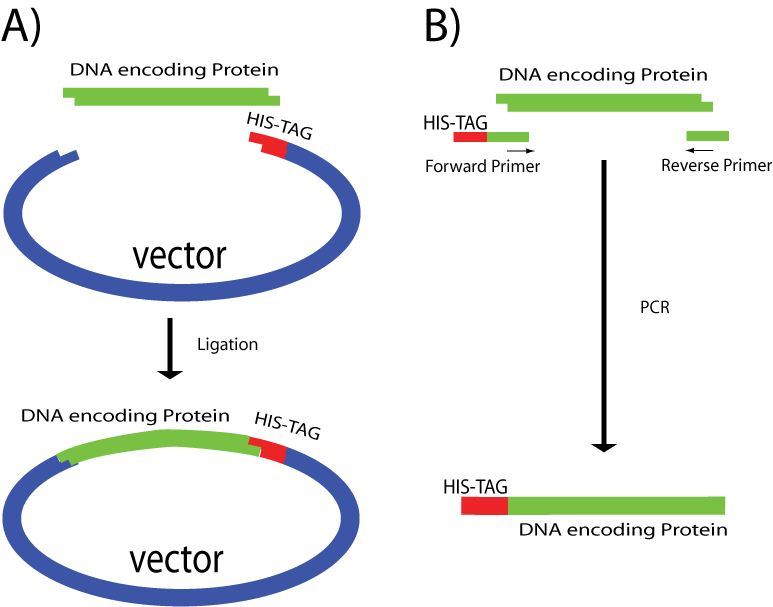
Protein purification with affinity tags, such as glutathione S-transferase (GST), histidine (HIS) and other affinity tags, allows the purification of proteins with both known and unknown biochemical properties. Therefore, this methodology has become a widely used research tool to determine the biological function of uncharacterized proteins. N-terminal 6xHis-GST-tagged Recombinant is a 211 amino acid (26 kDa) protein whose DNA sequence is frequently integrated into expression vectors for recombinant protein production.
The result of expression from this vector is a GST-tagged fusion protein in which the functional GST protein (26 kDa) is fused to the N-terminus of the recombinant protein. Because GST folds rapidly into a stable and highly soluble protein upon translation, the inclusion of the GST tag often promotes greater expression and solubility of recombinant proteins than expression without the tag. Additionally, GST-tagged fusion proteins can be purified or detected based on the ability of GST (an enzyme) to bind to its substrate, glutathione (GSH).
GST fusion protein purification
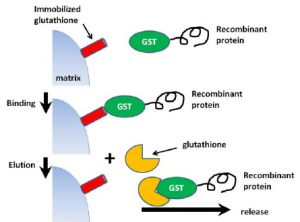
Glutathione is a tripeptide (Glu-Cys-Gly) that is the specific substrate for glutathione S-transferase (GST). When reduced glutathione is immobilized via its sulfhydryl group on a solid support, such as cross-linked bead agarose, it can be used to capture pure GST or GST-tagged proteins through enzyme-substrate binding reaction.
Binding is most efficient in near-neutral buffers (physiological conditions) such as Tris-buffered saline (TBS) pH 7.5. Because binding depends on the preservation of the essential structure and enzymatic function of GST, protein denaturants are not compatible.
After washing an affinity column to remove unbound sample components, the purified GST fusion protein can be dissociated and recovered (eluted) from a glutathione column by the addition of excess reduced glutathione. Free glutathione competitively displaces the binding interaction of immobilized glutathione with GST, allowing the fusion protein to emerge from the affinity column.
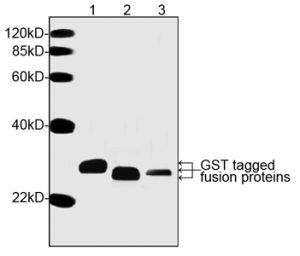
This affinity system typically produces greater than 90% pure GST-tagged recombinant protein from crude bacterial or mammalian cell lysate samples. Glutathione-based affinity purification of GST-tagged fusion proteins is easily performed on a small, medium, or large scale to produce microgram, milligram, or gram quantities.
At 26 kDa, GST is considerably larger than many other fusion protein affinity tags. For reasons that have not been fully characterized in the literature, the GST fusion tag structure is often degraded upon denaturation and reduction for protein gel electrophoresis (eg, SDS-PAGE). As a result, electrophoresed samples of GST fusion proteins often appear as a ladder of lower MW bands below the full-size fusion protein.
When the GST tag is not required or desired as part of the recombinant protein after purification, it can be removed if a cleavage site for a specific protease is included between the protein and the GST tag in the DNA vector design. . For example, the HRV 3C protease specifically cleaves the sequence Leu-Glu-Val-Leu-Phe-Gln-↓-Gly-Pro