For the development of new immunotherapies, it is essential to research the tumor microenvironment. Immunohistochemistry is a versatile technique for studying the expression, distribution and activation of proteins in situ. For the detection of antigens, specific antibodies are used on thin cross-sections made of shock-frozen or formaldehyde-fixed, paraffin-embedded tissue. The visualization of the antigen is made possible by enzymatic reactions which lead to the precipitation of dye at the site of the antibody-antigen binding, or by fluorescent reporters. Fluorescent reporters can either be conjugated directly to the primary antibody (direct immunofluorescence) or to a secondary antibody that recognizes the species-specific primary antibody (indirect immunofluorescence). The last variant is the more frequently used method, since it still delivers signals with very small amounts of antigen.
In the past, this technique was used individually for each immune marker. Recently, molecular histopathology has evolved from single marker immunohistochemistry to multiplex marker detection. Multiplex immunohistochemistry, also called multiple immunolabeling or multiplex immunostaining, can maximize the amount of data obtained from a single sample. This is particularly important in cases where the amount of sample is limited, such as a tumor biopsy. In contrast to next generation sequencing or mass spectrometry, the spatial arrangement of proteins as well as protein interactions and co-localization can be examined in multiplex immunohistochemistry.
Chromogenic detection is in principle compatible with multiplex immunohistochemistry, but indirect labeling techniques with tyramide-based fluorescence have several advantages. The signal is amplified by the deposition of fluorophore-conjugated tyramide at the antigen binding site. Tyramid Signal Amplification (TSA) enables the detection of very rare targets in a sample and improves the fluorescence signal. It is also important that TSA also allows the use of unlabeled primary antibodies with the flexibility to use multiple antibodies from the same species.
Another not insignificant advantage of tyramide-based fluorescence detection is the resistance of the tyramide-antigen binding, which takes place through the covalent binding of tyrosine residues on or near the antigen. This resistance allows the removal of antibodies using heat, while maintaining the fluorescent signal of the antigen. With this method, different antibodies from the same host species can be used in succession without the otherwise expected cross-reactions. If additional, non-overlapping excitation and emission wavelengths are selected, it is easy to visualize different target proteins at the same time. Multispectral recordings allow the graphic isolation of fluorophores with partially overlapping spectra and the reduction of tissue autofluorescence.
The latest advances in multiplex immunohistochemistry and multispectral imaging enable the precise and simultaneous analysis of multiple tissue markers. For example, the analysis of proliferation and autophagy markers in the intestinal tissue can help with an accurate assessment of the epithelium turnover. In clinical application, the elucidation of the tumor microenvironment and the selection of targeted therapies can be improved by localizing and examining immuncheckpoint proteins. The applications of multiplex immunohistochemistry are versatile and include clinical, translational and basic research.
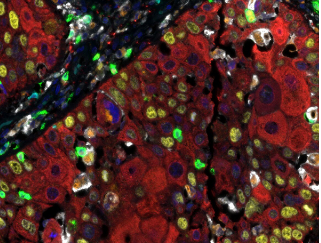
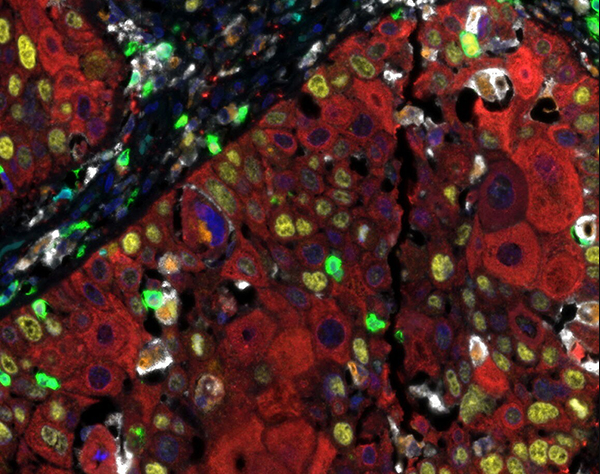
Detection of human CD3 (turquoise), CD8 (green), CD68 (orange), CK (red), Ki67 (yellow) and PD-L1 (white) in FFPE HNSCC with IHC-IF. Rabbit anti-CD3e recombinant monoclonal [BL-298-5D12], rabbit anti-CD8a recombinant monoclonal [BLR044F], mouse anti-CD68 monoclonal [KP-1], mouse anti-cytokeratin monoclonal [AE1 / AE3], rabbit anti-Ki67 monoclonal [BLR021E] and rabbit anti-PD-L1 recombinant monoclonal [BLR020E]. Secondary antibodies: HRP-conjugated goat anti-rabbit IgG and HRP-conjugated goat anti-mouse IgG. Substrate: Opal ™ 480, 520, 570, 620, 690 and 780. Counterstain: DAPI (blue).
Detection of human CD3 (yellow), CD8 (red), and CD20 (green) in FFPE tonsil by IHC-IF.
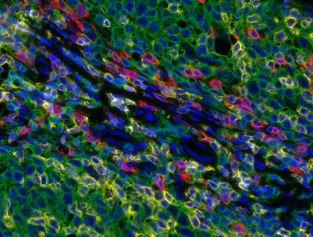
Detection of human CD3 (yellow), CD8 (red), and CD20 (green) in FFPE Mundel tissue with IHC-IF. Rabbit anti-CD3e recombinant monoclonal [BL-298-5D12], rabbit anti-CD8a recombinant monoclonal [BLR044F], mouse anti-CD20 monoclonal [L26]. Secondary antibodies: HRP-conjugated goat antirabbit IgG and HRP-conjugated goat anti-mouse IgG. Substrate: Opal ™ 520, 620, and 690. Counterstain: DAPI (blue).
Detection of human CD3 (yellow), CD20 (green), and CD68 (red) in FFPE lung carcinoma by IHC-IF.
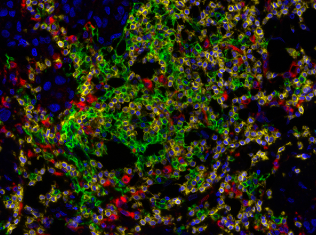
Detection of human CD3 (yellow), CD20 (green), and CD68 (red) in FFPE lung cancer tissue with IHC-IF. Rabbit anti-CD3e recombinant monoclonal [BL-298-5D12], mouse anti-CD20 monoclonal [L26], and mouse anti-CD68 monoclonal [KP-1]. Secondary antibodies: HRP-conjugated goat antirabbit IgG and HRP-conjugated goat anti-mouse IgG. Substrate: Opal ™ 520, 620, and 690. Counterstain: DAPI (blue).
Required reagents
Component product
PathPlex ™ antibody panels
PathPlex ™ Panel 1 (CD3E, CD8 alpha, PD-L1)
PathPlex ™ Panel 2 (CD3E, CD8 alpha, CD20)
PathPlex ™ Panel 3 (CD3E, CD68, CD20)
PathPlex ™ Panel 4 (CD3E, Cytokeratin, CD8 alpha, CD68, PD-L1, FOXP3)
PathPlex ™ Panel 5 (CD3E, Cytokeratin, CD8 alpha, CD68, Ki-67, PD-L1)
PathPlex ™ Panel 6 (CD3E, Granzyme B, CD8 alpha, cytokeratin, Ki-67, SOX10)
PathPlex ™ Panel 7 (CD8 alpha, cytokeratin, CD45RO, CD4, FOXP3)
PathPlex ™ Panel 8 (CD3E, Cytokeratin, CD8 alpha, CD4, LAG3, FOXP3)
HRP-conjugated secondary antibodies (depending on primary antibodies)
Anti-Mouse IgG-heavy and light chain, HRP conjugated
Anti-Rabbit IgG-heavy and light highly cross-adsorbed, HRP conjugated
Opal dyes
Opal Polaris 480 (Akoya Biosciences)
Opal 520 (Akoya Biosciences)
Opal 540 (Akoya Biosciences)
Opal 570 (Akoya Biosciences)
Opal 620 (Akoya Biosciences)
Opal 650 (Akoya Biosciences)
Opal 690 (Akoya Biosciences)
Opal Polaris 780 (Akoya Biosciences)
1x Plus Amplification Diluent
FP1498 (Akoya Biosciences)
DAPI
DAPI. dihydrochloride (AdipoGen Life Sciences)
Advantages of fluorescence multiplex immunohistochemistry using TSA
Fluorescence multiplex immunohistochemistry (mIHC) with tyramide signal amplification (TSA) has several advantages over one-color or traditional mIHC. The advantages below show what makes the mIHC with TSA a powerful technology for the visualization of several interesting target structures:
Visualization of multiple target structures within a single tissue section
This is crucial for experiments where the amount of sample is limited, e.g. for tumor biopsies or other clinical samples. mIHC enables the acquisition of the maximum amount of data from a single sample.
Investigation of the spatial arrangement of the target structures
The visualization of multiple targets within a single tissue section enables the spatial arrangement of the structures to be examined. This leads to a better understanding of protein interaction or co-localization within the conserved tissue architecture. This type of investigation is not possible with other techniques such as polymerase chain reaction, mass spectrometry or next-generation sequencing.
Further dynamic and linear measuring ranges
Compared to chromogenic detection, fluorophore detection offers a wider dynamic and linear measurement range, making it easier to visualize both high and low frequency targets on the same slide. The use of TSA also enables the signal amplification of targets with low frequency by amplifying the antigen-associated fluorescence signal.
Simplified panel design
The permanent existence of the covalent tyramide-tyrosine bond facilitates the heat-mediated removal of primary / secondary antibody pairs without interrupting the fluorescence signal. This means that any primary antibody validated for IHC, regardless of the host species, can be used for any target structure, as long as a specific secondary antibody is used.
Use of DAPI as counterstaining
Counterstaining the DNA with DAPI is preferable to hematoxylin because hematoxylin can be masked by other targets during chromogenic staining.
Spectral segregation
Spectral segregation ensures that the signals of each individual target structure can be distinguished from those of the other target structures. It also enables the subtraction of the signal caused by the autofluorescence of the tissue.
Rationalized quantification
The objective determination of the expression level of several targets can be standardized through the use of a suitable imaging platform and software. Since the tissue architecture is retained in the mIHC, the visualization of tissue reference points can also contribute to precise quantification.
Taken together, these features of fluorescent mIHC with TSA represent a robust approach to tissue analysis. Such analysis enables a variety of applications, such as the characterization of molecular signaling pathways, protein-protein interactions, the elucidation of the complex microenvironment of the tumor or the development of individually tailored therapeutic interventions.
